This document guides how to safely prescribe, administer and manage
patients receiving intravenous immunoglobulin (IVIg) at the RCH.
Description
- IVIg is
manufactured from human plasma. It contains IgG antibodies and is used in the
treatment of a growing number of immune, haematology, neurology and
rheumatology conditions.
- All IVIg products undergo several steps to minimise the possibility of pathogen transmission.
Authorisation to administer IVIg
- The
use of IVIg in Australia is governed by the National Blood Authority (NBA) and is issued by the Australian Red Cross Lifeblood.
- The Criteria for the clinical use of immunoglobulin
in Australia (the Criteria) outlines conditions where IVIg has been established to be approved and
access to IVIg is based on the patient meeting specific requirements as listed in the Criteria.
- IVIg
authorisations are processed via an online system called BloodSTAR. All
treating medical staff at RCH must register with BloodSTAR to request IVIg. Medical
staff need to register at each hospital they prescribe IVIg. To be eligible to
register at RCH an APHRA number and RCH email address are required. For assistance
registering call 13 000 BLOOD (13 000 25663).
- For any
request the clinician will require:
- Patient
demographics
- Diagnosis
- Patient
weight
- The dose
(g) of IVIg prescribed will vary according to the indication
- Consent -
BloodSTAR requires a specific ‘Record of privacy consent’. This indicates that the
patient understands their personal information will be recorded in BloodSTAR.
- For any medical conditions that do not meet the
criteria for funded IVIG under the National Blood Arrangements, an application
to the Drug Usage Committee (DUC) for hospital funded IVIg (Issued via
Pharmacy) may be made. There should be careful consideration for approval
balancing potential side effects with any possible alternate treatments.
- Nursing staff can register in the same process
as above and can view patient information related to IVIg authorisations,
previous and planned treatments, planning sheets and the record of privacy
consent.
IVIg
products available in Australia
Consent and patient information
- Document the consent for the administration of
IVIg on the Patient Consent to Blood Products
MR634/A. This is
separate from the BloodSTAR consent and is specific to RCH.
- Inform the patient of:
- The reason
why IVIg is needed, any risks of not receiving it and any potential alternate
treatments.
- The risks
and benefits of IVIg and potential side effects (e.g. acute reactions and
delayed adverse events including aseptic meningitis).
- Consent is
valid for the duration of the admission. However, consent for patients
receiving regular IVIg is valid for 12 months. This should be indicated on the
MR634/A.
- Ask
patients and families:
- To alert
nursing staff/treating team of any adverse event during or
following their IVIg infusion.
Contraindications
- True anaphylactic reactions to human
immunoglobulins
- IgA deficient patients with anti-IgA
antibodies
-
Flebogamma 5% and 10%
is contraindicated in patients with hereditary problem of fructose intolerance.
Babies and young children (< 2years) may not yet be diagnosed with this
condition (which may be fatal) and should not receive this product.
Precautions
- Reactions and adverse events to IVIg may relate to:
- Higher infusion rates (e.g., >3mL/kg/hr)
- Patients receiving higher doses of IVIg (e.g., 2g/kg)
- Patients naive to IVIg
- When changing from one IVIg product to another
- When there has been a long interval between infusions.
- Ensure patients are well hydrated prior to IVIg.
- Patients with a previous severe adverse event to IVIg (e.g., aseptic menangitis)
- Patients with history of or current cerebral oedema.
- IgA deficient patients
- Renal impairment
- Assess renal function prior to product commencement.
Special considerations
IVIg infusion may
impair the efficacy of live attenuated virus vaccines such as measles, rubella,
mumps and varicella and there are specific deferral periods.
|
Indication
|
|
Dose g/kg
|
Deferral
interval
|
|
IVIg for treatment of immune
thrombocytopenic purpura (ITP)
|
IV
|
0.4g/kg
|
8 months
|
|
IVIg for treatment of ITP
|
IV
|
1g/kg
|
10 months
|
|
IVIg
for treatment of ITP or Kawasaki
disease
|
IV
|
1.6 – 2g/kg
|
11 months
|
https://immunisationhandbook.health.gov.au/contents/vaccination-for-special-risk-groups/vaccination-for-people-who-have-recently-received-normal-human-immunoglobulin-and-other-blood-products
Medical order
- Order IVIg
in EMR.
- Type of
IVIg product and concentration (e.g., Privigen AU, Privigen 10%, Flebogamma 5%,
Flebogamma 10%, Kiovig 10%, Gammunex 10% and Octagam 10%)
- The IVIg
product approved in BloodSTAR will need to be the IVIg product prescribed in
EPIC.
- Dose (g)
- Rate of
infusion – this will include the starting rate, the incremental changes and
maximum rates.
- Pre-hydration
- Ensure
patients are well hydrated prior to IVIg infusion, consider hydration with 0.9%
saline (10ml/kg bolus).
- Pre-medications
-
Consider anti-pyretics (e.g., Paracetamol),
anti-histamines (e.g. Cetirizine) or anti-emetics (e.g. Ondansetron) if prior
rate-related reactions to IVIg.
Dose
- The dose
(g) of IVIg prescribed will vary according to the indication.
- Please
prescribe the dose authorised by BloodSTAR. BloodSTAR will approve a dose that
is rounded to the nearest vial size to reduce wastage.
-
In neonates, the dose
in g/kg may be smaller than the minimum vial size of 5g, and an aliquot should
be prescribed.
IVIg product descriptions and comparisons
|
Description
|
Privigen
AU* 10%
(Australian)
|
Privigen
10%
(Imported)
|
Kiovig
10%
(Imported)
|
Gammunex
10%
(Imported)
|
Octagam
10%
(Imported)
|
Flebogamma
10% DIF
(Imported)
|
Flebogamma
5% DIF
(Imported)
|
|
Concentration
|
10%
|
10%
|
10%
|
10%
|
10%
|
10%
|
5%
|
|
Presentation
|
5g in 50 mL
10 g in 100 mL
20g in 200 mL
|
2.5g in 50 mL
5g in 100 mL
10g in 200 mL
20g in 400 mL
|
|
Stabilising
agent
|
Proline
|
Proline
|
Glycine
|
Glycine
|
Maltose
|
Sorbitol
|
Sorbitol
|
|
IgA
level
|
< 0.025
mg/mL
|
< 0.025
mg/mL
|
<0.14 mg/mL
|
≤0.084
mg/mL
|
≤0.4 mg/mL
|
<0.1 mg/mL
|
< 0.05 mg/mL
|
|
Storage*
(All
products stored in blood bank)
|
Below 25°C
|
2 – 8°C*
|
Below 30°C
|
|
Compatible
fluids
|
0.9% Normal
Saline – may be used to flush IV-line post infusion
|
|
Consumer
product information
|
Privigen AU
|
Privigen
|
Kiovig®
|
Gammunex
|
Octagam
|
Flebogamma 10% Flebogamma 5%
|
IVIg administration rates
- Administration rates vary between 5% and
10% products.
- Patients receiving IVIg for the first time or switching IVIg
brands, should commence at the slowest rate and grade up according to the
table provided below.
- Infusion
rates should be individualised to the patient’s risk factors, comorbidities and
tolerability with previous infusions.
- Patients
at risk for Aseptic Meningitis Syndrome (AMS), renal failure or thromboembolic
adverse events should have IVIg administered at the minimum rate of infusion
practicable.
First infusion
- Use this
rate for:
- First infusion
- Switching between IVIg products
- Significant gap between infusions.
- The first
infusion will run slowly.
|
Description
|
Privigen
AU* 10%
(Domestic)
|
Privigen
10%
(Imported)
|
Kiovig
10%
(Imported)
|
Gammunex
10%
(Imported)
|
Octagam
10%
(Imported)
|
Flebogamma
10% DIF
(Imported)
|
Flebogamma
5% DIF
(Imported)
|
|
Commencement
rate (initial infusion)
|
0.25 mL/kg/hr
|
0.5 mL/kg/hr
|
1 mL/kg/hr
|
|
Incremental
rates:
increase
every 30 minutes only if tolerated
|
0.5 mL/kg/hr
1.0 mL/kg/hr
1.5 mL/kg/hr
2.0 mL/kg/hr
2.5 mL/kg/hr
|
1 mL/kg/hr
2 mL/kg/hr
3 mL/kg/hr
|
2 mL/kg/hr
4 mL/kg/hr
6 mL/kg/hr
|
|
Maximum
rate for first infusion
|
Do not exceed 150 mL/hr or 2.5 mL/kg/hr |
Do
not exceed 300 mL/hr or 6 mL/kg/hr |
Subsequent infusions
Only follow this
process if no adverse event to the first infusion (e.g., absence of headache,
fever, tachycardia, nausea, vomiting, pain, or significant BP or heart rate
change during and within 72 hours of first infusion).
|
Description
|
Privigen
AU* 10%
(Domestic)
|
Privigen
10%
(Imported)
|
Kiovig
10%
(Imported)
|
Gammunex
10%
(Imported)
|
Octagam
10%
(Imported)
|
Flebogamma
10% DIF
(Imported)
|
Flebogamma
5% DIF
(Imported)
|
|
Commencement
rate (subsequent infusion)
|
0.5 mL/kg/hr
|
1 mL/kg/hr
|
|
Incremental
rates:
increase
every 30 minutes only if tolerated
|
1.0 mL/kg/hr
1.5 mL/kg/hr
2.0 mL/kg/hr
2.5 mL/kg/hr
|
1 mL/kg/hr
2 mL/kg/hr
3 mL/kg/hr
|
2 mL/kg/hr
4 mL/kg/hr
6 mL/kg/hr
|
|
Maximum
rate
|
Do not
exceed 240 mL/hr
|
Do
not exceed 300 mL/hr
|
Do
not exceed 600 mL/hr*
|
Foot Notes: * Please consider
infusion rate and patient comorbidities. Consider slower rates in patients
receiving doses of 2 g/kg.
Bottle sizes:
- Blood bank will issue some
smaller bottle sizes related to the dose the patient is ordered. This will
decrease the chance of a bottle expiring at 6 hours, before all the dose has
been administered.
- For example:
- 20g IVIg ordered. Blood bank will issue 2 x 10g bottles.
- 60g IVIg ordered. Blood bank will issue 2 x 10g bottles and
2 x 20g bottles.
- Please administer the smallest bottle first.
*Storage
- Please
note different storage temperatures for the different IVIg products (Table 1).
- Once
removed from blood bank store IVIg at room temperature. Do not place in fridges
in clinical areas.
-
If IVIg treatment is delayed for any reason
return the product to blood bank..
Administering and completing IVIg
- Allow IVIg to reach room temperature.
- IVIg does not contain any antimicrobial
preservative, therefore each bottle of IVIg must be administered within 6
hours of spiking the bottle.
- Use a vented system. Do not use a burette.
- Standard IV infusion giving set. IVIg
does not need to be filtered; however, filtering will not harm the
product.
- Dedicate an IV line – do not mix with
other medications or IV solutions (other than 0.9% saline which can be only be used post infusion to flush the IV line).
- Prime the IV infusion line with the IVIg
product.
- Ensure the correct infusion rate is
prescribed and programmed into infusion pumps.
- Commence with the smallest bottles first.
- Document rate changes in EMR.
- It is not necessary to flush between
bottles/batches.
- Flush with 0.9% Saline at conclusion of
the infusion to ensure the entire dose is received.
- Document the completion time and total
amount infused in the EMR.
-
During EMR downtime IVIg will be issued with a
compatibility report. Complete all details and send to HIS for scanning.
Requesting replacement/additional bottles for an incomplete IVIg dose:
Replacement
or additional bottles may be requested if the current bottle has been spiked
for 6 hours, or needs to be discarded, due to an adverse event or issues with
IV access.
- Calculate the ‘missing’ IVIg dose.
- 1g = 10 mL for all 10% IVIg products
- For example:
- 20g IVIg ordered = 200mL, patient received total of 150mL =
15g and ‘Missing’ dose is 5g
- Call blood bank on ext. 55829 and explain the issue.
- e.g., IVIg adverse event and infusion unable to be completed
at fast administration rate and bottled expired.
- Patient was ordered 20g and is ‘missing’ 5g of this dose.
- Request replacement/additional bottle to make up ‘missing’
5g dose.
- Reprint the EMR IVIg order and take to blood bank.
- NOTE: this will
state the original EMR order (e.g., 20g). Do not take receipt of the full
order, only the ‘missing’ dose. (e.g., 5g).
-
Only administer the ‘missing’ dose.
Observing
the patient receiving IVIg
| Time
point |
Observations
|
|
Baseline
(within 60 minutes before commencing transfusion) |
Temperature, pulse, respiratory rate, blood pressure, SaO2 |
| 15 minutes after commencing document observations & close visual observation for at least 30 minutes |
Temperature, pulse, respiratory rate, blood pressure, SaO2 |
| At each rate change | Temperature, pulse, respiratory rate, blood pressure, SaO2, rate and IV pump volume |
| Hourly until transfusion is completed |
Temperature, pulse, respiratory rate, blood pressure, SaO2, rate and IV pump volume |
| Conclusion |
Temperature, pulse, respiratory rate, blood pressure, SaO2, rate and IV pump volume.
Observe the patient post the infusion. |
- Ask the patient and parents to report any pain at the IV site, or symptoms during the infusion (e.g., headache, nausea, chills).
Adverse events
|
|
ADVERSE EVENTS
|
|
Acute (during
or within 6 hours of infusion)
|
|
Mild to moderate
|
Severe
|
|
Symptoms
|
- Chills/rigor
- Headache
- Nausea/vomiting
- Pain – abdominal, extremity, chest and back pain
- Rash/urticaria/flushing
- Dizziness
- Dyspnoea
|
- Anaphylaxis
- Transfusion
related circulatory overload (TACO)
|
|
Signs
|
- Fever
- Changes in BP or HR
- Changes
in RR or SaO2.
|
|
Management
|
- Pause IVIg
- Request
medical review
- Treat as appropriate (e.g., paracetamol,
anti-histamines or IV fluids)
- If the symptoms resolve and observations
stabilise then cautiously restart at previously tolerated rate
- Monitor patient closely
- If symptoms
return or escalate cease IVIg.
|
- Cease
infusion, follow MET/Rapid review process, treat as guided by symptoms (e.g.,
oxygen, adrenaline)
- Anaphylaxis is a rare but documented
adverse effect of IVIg. Please refer to the Anaphylaxis guideline for management
|
|
Future IVIg infusions
|
Consider pre-hydration (0.9% saline bolus) and/or pre-medication (e.g.,
paracetamol, antihistamine) for future IVIg infusions related to symptoms.
Consider capping
future IVIg infusions at tolerated rate.
|
|
REPORT ALL POTENTIAL
ACUTE OR DELAYED ADVERSE EVENTS (including mild events)
1. Call Blood bank on ext: 55829.
2. Order a “Transfusion Reaction evaluation” in EMR.
3. Document any adverse effects in EMR (e.g.,
headache, fever, nausea).
4. Blood bank staff will contact the on-call
haematologist who can provide support/advice for management of the current
IVIg infusion (e.g., medication to treat, hydration, slower infusion rates or
changing IVIg product) and future infusions.
5. Report any
other adverse event (e.g., administration issue) or near miss via VHIMS.
Adverse
events and near misses are discussed at the Blood Management Committee and
are reported to CSL as needed.
|
|
|
ADVERSE EVENTS
|
|
Delayed (6 hours to
72 hours post IVIg infusion)
|
|
Mild to moderate
|
Severe
|
|
Symptoms
|
- Headache
- Fever
- Nausea/vomiting
- Flu-like
illness
- Pain,
muscle aches or arthralgias
- Fatigue
- Rash
|
- Aseptic meningitis (*see below for further information)
- Typically
within 72 hours of IVIg
- Characterized
by fever, headache, altered mental status, nausea/vomiting, neck stiffness
and photophobia.
- CSF
evaluation not required for diagnosis.
- Increased
frequency in those with history of migraines
- Increased
frequency with high dose IVIg
- Thromboembolic
events
- Infusion
of IVIg may lead to increased blood viscosity
- Renal
impairment
- Haemolytic anaemia
(IVIg contains Anti-A and Anti-B, these can cause haemolysis and a
positive DAT, particularly in blood group A patients)
|
|
Management
|
- Treatment of
symptoms with simple measures (e.g., Paracetamol, anti-emetics).
|
- May need admission to
hospital
- See investigation below for
severe headache.
- IV fluids, supportive care
with analgesia (e.g., Paracetamol and Nurofen [if not contraindicated]) and
anti-emetics
- Seek haematology guidance.
|
|
Future IVIg infusions
|
Consider pre-hydration (0.9% saline bolus) and/or pre-medication (e.g.,
paracetamol, antihistamine for future IVIg infusions related to symptoms.
Consider capping
future IVIg infusions at tolerated rate.
|
|
REPORT ALL POTENTIAL ACUTE OR DELAYED ADVERSE EVENTS
(including mild events)
1. Call Blood bank on ext: 55829.
2. Order
a “Transfusion Reaction evaluation” in EMR.
3. Document any adverse effects in EMR (e.g.,
headache, fever, nausea).
4. Blood bank staff will contact the on-call
haematologist who can provide support/advice for management of the current
IVIg infusion (e.g., medication to treat, hydration, slower infusion rates or
changing IVIg product) and future infusions.
5. Report any other adverse
event (e.g., administration issue) or near miss via VHIMS.
Adverse events and near misses
are discussed at the Blood Management Committee and are reported to CSL as
needed.
|
|
* Management
of the patient with a severe headache (e.g., possible aseptic meningitis)
post IVIg
|
|
Symptoms
|
- Headache
- Nausea/vomiting
- Fever
- Altered mental status
- Neck stiffness
- Photophobia
|
|
Investigations
for consideration
|
- Venous gas
- Electrolytes
including calcium, magnesium and phosphate
- Ammonia
- Consider neuroimaging (e.g., CT or MRI) in discussion with the
reviewing doctor, considering the underlying diagnosis (e.g., ITP), presence
of neurological symptoms, and severity of symptoms.
- Lumbar puncture is not required to make the diagnosis of aseptic
meningitis.
|
|
Management
|
- May need
admission to hospital
- IV
fluids, supportive care with analgesia (e.g., Paracetamol and Nurofen [if not
contraindicated]) and anti-emetics
|
|
Seek
haematology advice if you suspect aseptic meningitis and report.
|
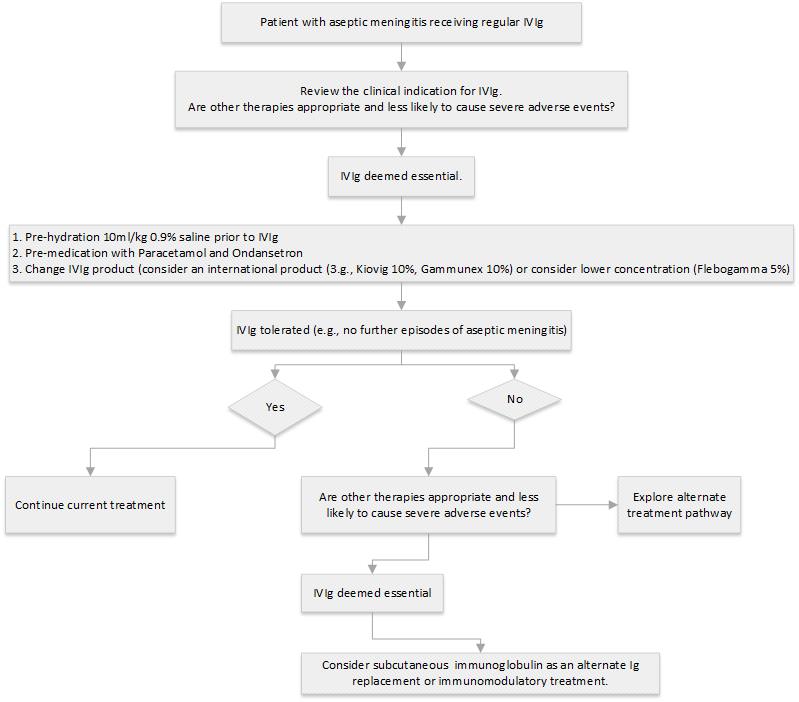
Management of patients with history of aseptic meningitis, requiring ongoing treatment with IVIg
Last updated July 2024