Introduction
Peripheral intravenous catheters (PIVCs) are the most commonly used invasive device in hospitalised paediatric patients. They are primarily used for therapeutic purposes such as administration of medications, fluids, and blood products.
Aim
The aim of this guideline is to provide evidence-based recommendations for the management of peripheral intravenous catheters (PIVCs), including midlines and extended dwell PIVCs. For information related to PIVC insertion, please refer to RCH
Clinical Practice Guideline: Intravenous access - peripheral.
Types of PIVCs
A peripheral intravenous catheter (PIVC) is a thin plastic tube inserted into a vein using a needle. PIVCs allow for the administration of medications, fluids and/or blood products. Some PIVCs have a longer catheter length, which are usually inserted
under ultrasound guidance. A longer PIVC is referred to as an extended dwell PIVC.

Image
1: a peripheral intravenous catheter (PIVC); Illustration by The Royal Children's Hospital, Melbourne
A midline is a type of peripheral intravenous catheter which is usually between 5-10cm long and inserted into the brachial or basilic veins in the upper arm. These devices are inserted by specially trained practitioners. A midline is to be managed
in the same way as a PIVC as the tip of the device remains in the peripheral vasculature.
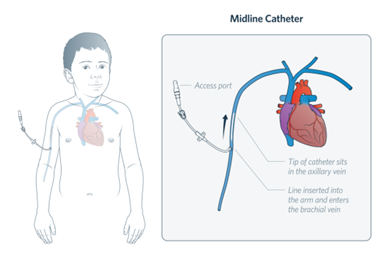
Image 2: a midline catheter
Definition of terms
- Aseptic technique aims to prevent pathogenic microorganisms from being introduced to susceptible sites by hands, surfaces and equipment. It is a set of practices designed to reduce contamination and protect the patient from infection during
invasive procedures such as midline/PIVC insertion and maintenance. See procedure here.
- Hand Hygiene is performed to protect the patient from organisms which may enter their key sites or key parts during a procedure. See procedure here.
- Key Parts are parts of the device/s that must remain aseptic throughout the clinical procedures. Examples of key parts include, the catheter hub, needleless connector, syringe hub and drawing up needle.
- Key Sites are the area on the patient such as a wound or intravenous insertion site that must be protected from microorganisms
- Scrub the hub ensure the needleless connector is ‘scrubbed’ vigorously with 2% chlorhexidine and 70% alcohol swab for 15 seconds and allow for it to completely air dry.
Assessment
Midline/PIVC site assessment aims to:
- Ensure early recognition of complications such as extravasation, phlebitis, occlusion and device associated pressure injuries.
- Ensure the dressing, catheter and attachments are intact and secure to prevent dislodgement.
- Maintain visibility of the insertion site.
How to perform midline/PIVC site assessment
Midline/PIVC assessment is to include the ‘Touch, Look, Compare’ technique (TLC). Whenever possible, cluster site assessment with other care.
-
Touch to assess the site is soft, warm, dry and non-tender.
- Look to ensure the site is dry and without redness. Ensure tapes are not too tight, there are no kinks in the catheter and the insertion site is visible.
- Compare the site with the other limb or side of the body. The site should be the same size, without swelling.
Indications for midline/PIVC
removal:
With each assessment, also assess clinical indication for ongoing access. If the patient no longer requires IV access, remove the device as soon as clinically appropriate to avoid complications.
Caregiver and patient education:
Education should be provided on the signs of injuries and the process of contacting nursing staff.
Frequency
of midline/PIVC assessment:
|
Risk
assessment
|
Frequency
of assessment
|
|
Critical care areas (paediatric intensive care unit and neonatal intensive care unit)
|
At least
every hour.
|
|
For patients receiving high-risk* medications, continuous infusions or large volume fluid boluses
|
At least
every hour.
See table below for list of high-risk medications.
|
|
Continuous IV fluid infusions (e.g., maintenance fluid with no additives or TKVO)
|
At least every four hours.
|
|
Continuous IV fluid infusions with intermittent medication administration
|
In addition to routine 4 hourly assessments (as above), assess the midline/PIVC site before and after each medication infusion.
|
|
Disconnected midlines/PIVCs
|
Every 8 hours. Whenever possible, try to cluster assessments with cares.
|
|
Wallaby (Hospital-in-the-Home) patients
|
The nurse will assess the midline/PIVC with each visit. Families receive education and support about checking midline/PIVC and communicating any concerns.
|
|
Clinical condition changes
|
The midline/PIVC site should be assessed if there is a change in the patient's condition, such as clinical deterioration or any signs of discomfort or escalating requirements for analgesics.
|
Documentation
- Midline/PIVC site assessment and complications are to be documented in the LDA flowsheet row.
- The volume of fluid infused, and the infusion rate is documented in the fluid balance flowsheet.
- Documentation occurs with each assessment.
Management of Midline/PIVC associated complications
|
Complication
|
Definition
|
Recognition
|
Management
|
|
Extravasation
(other common terms ‘tissued’ or ‘infiltration’)
|
Leaking of a fluid or medication into extravascular tissue from a midline/PIVC with potential to cause short- or long-term tissue damage.
|
Can present as pain, swelling, erythema, induration, blistering, pallor, blanching or catheter dysfunction.
|
For assessment and management of extravasation injuries, please see the Peripheral extravasation injuries initial
management and washout procedure CPG. |
|
Phlebitis
|
Is a sign of vessel damage. The cause can be chemical (due to the osmolarity of the solution), mechanical (from trauma at insertion or movement) or infective (microorganisms contaminating the device). Signs include swelling, redness, heat, induration,
purulence or a palpable venous cord (hard vein). Symptoms include pain related to local inflammation of the vein at or near the insertion site.
|
Can range in severity from slight pain and redness at the midline/PIVC insertion site to severe erythema, pain, swelling, tenderness and signs of infection (purulence, palpable vein cord).
|
If slight pain and redness are present, closely monitor the PIVC insertion site (at least hourly). If chemical phlebitis is suspected, consider altering the dilute of the infusate or slowing down the infusion rate and assess if symptoms resolve. If there
is an increase in pain, redness and swelling, remove the midline/PIVC. If severe phlebitis is present, consult the medical team as additional intervention may be required.
|
|
Occlusion
|
Is characterised by the inability to flush or administer fluid into the midline/PIVC.
|
Occlusion occurs when there is mechanical dysfunction (e.g., a kink in the catheter), a blockage (from blood components) or due to an extravasation injury. All causes of occlusion may affect catheter patency.
|
Patency can be evaluated by assessing response to infusing medication, ease of flushing, and by performing a TLC assessment. If a kink in the external portion of the catheter is visible, consider re-dressing the midline/PIVC. If catheter patency is not
restored, the midline/PIVC will need to be removed.
|
|
Dislodgment
|
Is the unintended removal of the catheter out of the vein.
|
There may be signs of midline/PIVC dysfunction (signs of occlusion or pain) and a portion of, or the entire catheter will be visible.
|
Using sterile gauze, apply pressure to the midline/PIVC insertion site (if bleeding) until haemostasis is achieved.
|
|
Device associated pressure injuries
|
Occur when the midline/PIVC hub, extension set, needleless connector or tapes are forced into the skin. Midline/PIVC associated pressure injuries can cause varying degrees of harm including superficial erythema to deep tissue damage.
|
May develop if any component of the midline/PIVC is forced into the skin. Pain, erythema and skin breakdown may be present.
|
Regular and thorough TLC assessment may reduce the risk of device associated pressure injury. TLC assessment is to include observing for erythema with any of the device components and relieving pressure by repositioning tapes or hard plastics
(Pressure Injury Guideline)
|
*High
risk medications
Please see Peripheral Extravasation Injuries CPG for list of high risk medications.
Management of Midline/PIVC
Anytime a Midline/PIVC is accessed please ensure the following occurs:
- Scrub the hub, needless connector is ‘scrubbed’ vigorously with 2% chlorhexidine and 70% alcohol swab for 15 seconds and allow for it to completely air dry.
- Aseptic technique is utilised, see Aseptic technique procedure.
Infusion Pump Pressure
Pressure limit defaults for intravascular infusion pumps are programmed by Biomedical Engineering, based on the manufacturer’s recommendations.
Upper limit infusion pump pressure can be manually increased with clinical discretion to accommodate:
- Increased viscosity of the fluid being administered
- High rate of the fluid being administered
- Reduced diameter of the intravascular catheter
- Increased length of the intravascular catheter
- Increased level of patient activity
If pump pressure exceeds the recommended limits, check the patency of the midline/PIVC.
Special consideration: Patients admitted to the Neonatal Unit should have line pressure documented within the Peripheral IV Cannula Lines, Drains, and Airway (LDA) tab.
Flushing and locking midlines/PIVCs
To keep vein open (TKVO)* is a continuous infusion of fluid administered in-between medication(s). The infusion rate of TKVO usually ranges from 1mL-10mL per hour. TKVO rates vary depending on the patients age, weight and underlying condition.
Intermittent locking* refers to the administration of a small volume (usually between 2-10mL) of 0.9% normal saline in-between infusions and then disconnecting the infusion set from the midline/PIVC. The volume of the lock should take into consideration
the volume of the midline/PIVC, and any add on devices. The lock is to be administered using a pulsatile (push-pause) technique and if a clamp is present, it is to be closed under positive pressure (i.e., whilst the lock is being administered).
*There is limited evidence available to guide practice on
the advantage of TKVO versus intermittent locking in patients who are not
receiving continuous medication or fluid. Intermittent locking may be the
preferred method for patients who are able to ambulate whilst receiving
intermittent intravenous treatment.
If the midline/PIVC is to be accessed intermittently for the administration of medications or fluids, it is to be flushed prior to infusion or at least once a shift
In most cases, 0.9% sodium chloride for injection is to be used to flush the midline/PIVC. This must be prescribed as a medication
Use 10mL syringe for flushing to avoid excessive pressure and catheter rupture. If resistance is felt during flushing and force is applied this may result in an infiltration or extravasation injury
Use aseptic non touch techniques including cleaning the access port (scrub the hub) vigorously for at least 15 seconds and allowing to dry prior to accessing the system
When flushing or locking the midline/PIVC use a pulsatile flushing technique (push pause motion)
Flush catheters:
- Before and after each infusion
- Immediately after placement
- Prior to and after fluid infusion (as an empty fluid container lacks infusion pressure and will allow blood reflux into the catheter lumen from normal venous pressure) or injection.
- Prior to and after blood drawing
Lock catheters:
- At the completion of an intermittent infusion;
- Close clamp or ensure 3-way tap is clamped.
Midline/PIVC dressings
Midline/PIVC dressings promote catheter security and prevent infection as they provide a barrier to the external environment. PIVC dressings are to be kept secure, clean, dry, intact and easily visible. The integrity of the dressing and all PIVC components
are to be assessed when preforming TLC.
There is no evidence available to suggest that routine PIVC dressing changes are beneficial. Dressing changes are to occur when either the insertion site or dressing are no longer secure, clean, dry and intact.
If the midline/PIVC requires a dressing change, consider the risk of the procedure and determine if a standard or surgical aseptic technique should be used.
Midline/PIVC removal
Midline/PIVC removal is to occur as soon as the device is no longer required.
Using a standard aseptic technique, gently remove the old dressing and then pull the catheter our of the skin whilst applying pressure (with gauze) to the insertion site until haemostasis is achieved.
Document removal in the medical record.
Companion Documents
RCH Clinical Practice Guidelines
RCH Nursing Guidelines
RCH Policies and Procedures
Evidence Table
| Reference |
Evidence level
|
Key
findings, outcomes or recommendations |
Australian Commission on Safety and Quality in Health Care (2021) Management of Peripheral Intravenous Catheters Clinical Care Standard
|
VII |
Care standards state:
A patient with a PIVC will have it removed when it is no longer needed or at
the first sign of malfunction or local site complications. Clinical indication
replacement may also reduce discomfort for patients associated with regular
placement.
The device is secured using a sterile, transparent, semipermeable dressing
unless contraindicated. Ensure that the dressing remains intact for the
duration of the insertion to prevent complications i.e., unintended
dislodgement.
|
Corley, A., Marsh, N., Ullman, A. J., & Rickard, C. M. (2022). Peripheral intravenous catheter securement: An integrative review of contemporary literature around medical adhesive tapes and supplementary securement products. Journal of Clinical Nursing
|
I
|
The systematic review found that any product used directly at the PIVC insertion site should be sterile. As non-sterile tape directly over the PIVC site is associated with poor PIVC outcomes. Splints and arm boards can be used to immobilise PIVCs, placed at point of flexion such as the wrist or antecubital fossa. However, no studies testing splints or arm boards were identified in the literature. Guidelines recommended tubular over rolled bandages. If a bandage is used to cover the PIVC site, it must be easily removed by nursing staff to perform regular site assessments.
|
Flint, A., & Davies, M. (2008). The intravenous cannula for newborn infants requiring only intravenous medication: continuous infusion or intermittent flushing? Journal of Infusion Nursing, 31(6), 346-349.
|
IV |
|
Gorski, L. A., Hallock, D., Kuehn, S. C., Morris, P., Russell, J. M., & Skala, L. C. (2012). Recommendations for frequency of assessment of the short peripheral catheter site. Journal of Infusion Nursing, 35(5), 290-292.
|
VII |
Assess
PIVC site hourly for paediatric patients.
Site
assessment should include redness, tenderness, swelling, drainage, and/or the
absence of parathesis, numbness or tingling. Assessment involves visual
assessment, palpation and subjective information from the patient.
*Please note that no
further studies on PIVC site assessment were identified and their
recommendation for hourly site assessment was published more than 10 years ago.
Our guideline has been updated to reflect current practices, based on clinical
consultation and local expert consensus.
|
Nickel, B., Gorski, L., Kleidon, T., Kyes, A., DeVries, M., Keogh, S., ... & Hagle, M. E. (2024). Infusion therapy standards of practice. Journal of Infusion Nursing, 47(1S), S1-S285.
|
VII |
Recommendations
include:
Assess
and discuss daily need of PIVC with treating team.
A
single tubular sleeve is preferred to a rolled bandage if additional security
is required.
Use
physical immobilisation devices for paediatric patients. Use in a manner that
permits visual inspection and assessment of the vascular access site and
pathway and does not exert pressure that will cause pressure injuries.
Flush
PIVC with 0.9% sodium chloride using a pulsatile technique.
Use a minimum volume
equal to twice the internal volume of the catheter system (catheter plus add on
devices). Larger volumes (5mL for PIVC) may remove fibrin deposits, drug
precipitate, and other debris from the lumen.
|
| Kleidon, T. M., Keogh, S., Flynn, J., Schults, J., Mihala, G., &
Rickard, C. M. (2020). Flushing of peripheral intravenous catheters: A pilot,
factorial, randomised controlled trial of high versus low frequency and volume
in paediatrics. Journal of Paediatrics and Child Health, 56(1),
22-29. |
II |
The RCT
was a pilot study to inform research protocol and sample size calculations for
a definitive trial.
PIVC
failure was significantly associated with decreased flushing volume, suggesting
flush volume is associated with PIVC failure in paediatric patients.
Group comparisons for
flush frequency indicated that once daily flushing was as good as 6-hourly
flushing. Overall analysis of the pilot suggested clinicians could use 10mL
flushing volumes every 24 hours; with no PIVC failure or cost.
|
| Stok, D., & Wieringa, J. W. (2016). Continuous infusion versus
intermittent flushing: maintaining peripheral intravenous access in newborn
infants. Journal of Perinatology, 36(10), 870-873. |
IV |
Full term newborn infants receiving IV amoxicillin and
gentamicin
TKVO: n=48
Saline lock: n=50
Primary outcome - PIVC patency No significant difference between groups
TKVO = median dwell 57.48 hours
Saline lock = median dwell 55.42 hours
|
| Webster, J., Osborne, S., Rickard, C. M., & Marsh, N. (2019).
Clinically‐indicated replacement versus routine replacement of peripheral venous
catheters. Cochrane Database of Systematic Reviews, (1). |
I |
The
systematic review demonstrated moderate to low certainty evidence of no clear
difference in rates of catheter-related bloodstream infection and
thrombophlebitis, between clinically indicated or routine replacement.
Moderate
certainty evidence that clinically indicated removal probably reduces
device-related costs.
Results indicate that
healthcare organisations should consider policy recommendations that state
catheters are changed only if there is a clinical indication to do so.
|
| Yeung, F., Miller, M. R., Ojha, R., McKelvie, B., Poonai, N., Bock, D.
E., ... & Taheri, S. (2020). Saline-lock versus continuous infusion:
maintaining peripheral intravenous catheter access in children. Hospital
Pediatrics, 10(12), 1038-1043. |
IV |
Prospective, time-allocated, cohort study comparing TKVO
versus intermittent saline locks in two three-month blocks (Canada)
TKVO: n=85
Saline lock: n=87
Primary outcome - PIVC patency
No significant difference between groups
TKVO = median dwell 57.48 hours
Saline lock = median dwell 55.42 hours
|
Please remember to read the
disclaimer.
The development of this nursing guideline was coordinated by Eloise Borello, CNC Vascular Assess Specialist Team approved by the Nursing Clinical Effectiveness Committee. Updated March 2025.